India

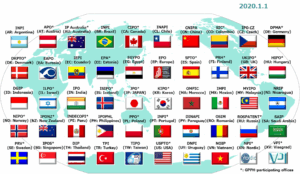
PPH network reaches over 50 members as India and Saudi Arabia join
By Troels Peter Rørdam | Posted on February 3, 2020
The ever-expanding Patent Prosecution Highway (PPH) network has gained two new additions. AWA Point sums up the new members and opportunities. Two new authorities with large economies have entered the PPH, providing the ability to speed-up and simplify examination of patent applications. The new additions are India and Saudi Arabia. For a given PPH-agreement,...
Tags: applications, India, patents, PPH, prosecution, Saudi Arabia
The Indian Jungle – an IP perspective, part 2
By Michael Bech Sommer | Posted on March 10, 2015
To foreign applicants filing a patent application in India takes place “at the IPO”, but in reality there is a choice of venue which is not trivial. The Indian patent office (IPO) is headquartered at Kolkata (formerly known as Calcutta) with branches in Chennai (formerly Madras), New Delhi and Mumbai (formerly Bombay). The office of...
Tags: compulsory license, India, Patent
The Indian Jungle – an IP perspective, part 1
By Michael Bech Sommer | Posted on February 27, 2015
“Now these are the laws of the jungle, and many and mighty are they…” – Rudyard Kipling wrote these lines over 100 years ago in his “Law for the Wolves” from the famous Jungle Book. As IP practitioners we are constantly reminded of the complex nature of today’s Indian IP jungle and the many and...
Poverty versus patenting in India – round two
By Jeanette Jakobsson | Posted on November 22, 2012
Earlier this year the first compulsory license, regarding the anti-cancer drug Sorafenib, was granted in India. (See previous blog post.) The compulsory license was granted inter alia on the ground that the patented invention was not available to the public at a reasonable affordable price. The battle towards affordable medicaments in India continues. In a...
Tags: compulsory license, drug patents, India, Pharmaceuticals
India Grants the First Compulsory License
By Jeanette Jakobsson | Posted on March 30, 2012
The Compulsory License Application No. 1 of 2011, the first of its kind in the history of the Indian Patents Act 1970, concerns the anti-cancer drug Sorafenib, where the patentee is Bayer Corporation and the applicant for a compulsory license is Natco Pharma Limited. The anti-cancer drug Sorafenib, sold under the brand name NEXAVAR, is...
Recent contributors

Partner, Attorney at Law, Business Area Manager

Partner, European Patent Attorney

Senior Counsel, European Patent Attorney

Counsel, AU and NZ Patent Attorney

Senior Associate, China Patent Attorney

Senior Associate, Attorney at Law